My earliest memories are from Gamboa, a little town in a tropical rainforest, where the Chagres River flows into the Panama Canal. Gamboa is in many ways a jungle paradise. But it is also home to the Panama Canal Dredging Division where my grandpa Charlie worked as a drill boat captain. The dredging division’s work resembles a military operation. The mountainous terrain near Gamboa—a highly unstable goo of volcanic and sedimentary rock often drenched by torrential rains—is regularly trying to reclaim the shipping channel. Charlie and his crew blew up rocks for a living, all to the purpose of keeping the canal open to ship traffic. He was really good at it.
My awareness of these and other long-running battles against nature accrued over time. There was a French graveyard not far from our house, the crosses with their backs to the jungle, the tombstones bearing the names of those who’d died (mostly from malaria and yellow fever) during France’s disastrous effort to build a canal in the late 19th century. My favorite fishing spot, as I got to grade school, was a different kind of memorial—a rusting, French ladder dredge, half-buried in the mud at the Diablo Spinning Club. To my 10-year-old eyes it looked like it had been there forever.
To the extent we talked about nature, it was usually with an emphasis on the control of nature and protecting ourselves from insects, infections, and venomous bites. My church taught that we were eternal beings; that our time, here, would be relatively brief and preparatory to an eternal after-life—if we behaved ourselves. Until then, Earth was our sandbox—a place that existed as the physical stage for our mortal lives. Heaven was somewhere else and the stars in the night sky—so impossibly far away—were enchanting but useful only to poets and songwriters. It turns out the stars are much more involved in our lives than I could have imagined.
The nature of heaven remains open for metaphysical debate, but not the stars. My attraction to rocks—my interest in geology—goes back to childhood. By far, the most compelling geologic fact is that expiring and exploding stars not only provided the raw materials for our crusty blue planet and its rocks; they are the source of the elements that make us. That’s true not just poetically, but literally.
The stars may be so distant as to challenge our imaginations. But we now know, for certain, they are the intensely broiling forges that create the heavier elements that make our lives possible—i.e. carbon for our genes, iron for our hemoglobin, calcium for our teeth and bones, iodine to regulate our growth and metabolism, zinc for scores of vital enzymes. In bulk, oxygen and carbon—elements that are only produced by the“nucleosynthesis” in dying stars—constitute more than 80 percent of our body’s mass.
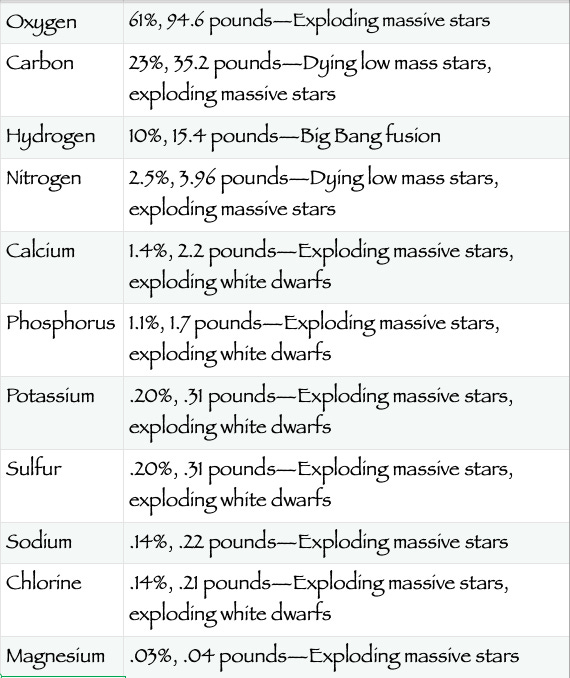
The table below shows, in percentage mass and actual weight (in Earth gravity), the abundance of star-created ingredients in a typical, 154-pound human, and the stellar events that created the respective elements.
(Source credits: Anne Marie Helmenstine, Elemental Composition of the Human Body by Mass, and for star death creation, Jennifer Johnson, Ohio State University Astronomy department, The Origin of the Elements.)
After magnesium, there are 48 additional, star-furnace elements (including iron, zinc, iodine and gold) that, individually, make up less than an ounce in a 154-pound body.
The discovery of this deeply profound connection between us and the stars arrived incrementally over the past half century. There was no single moment—like a moon landing or an Olympic flame lighting—that focused the world’s attention to it. It is a mind-bending story that just sort crept up on us, or at least most of us.
The prolific scientist Carl Sagan had written about the stardust-to-humans phenomenon in a 1973 book. But this was before he emerged as a popular culture celebrity as the host for the popular PBS nature series Cosmos in 1980. Sagan repeated his statement “we are star stuff” for the Cosmos audience, to which he added “we are a way for the Cosmos to know itself.” The second phrase is whimsical and, perhaps inadvertently, left many in his audience to wonder whether he was speaking figuratively about all of it. That said, he was clearly speaking literally when he said humans are physical manifestations of “star stuff.”
The deep time and astrophysics behind our human table of ingredients invite gasps. The scientific consensus is that the “Big Bang” that birthed the universe occurred nearly 14 billion years ago. Theories abound as to what caused it, but astrophysicists have much better ideas and evidence of what began to happen within billionths of a second after the primordial explosion. For starters, there is broad agreement that the Big Bang was extremely hot—an estimated 1,000 trillion degrees celsius.
For the first 380,000 years after the Big Bang the universe was simply too hot for electrons to become attached to atomic nuclei. As the heat subsided, and electrons settled around nuclei, the first stable elements began to form: hydrogen, helium, and relatively small amounts of lithium and deuterium—the lightest of elements. Hydrogen (one electron and one proton) and helium (two electrons and a nucleus of either two protons or one proton and a neutron) eventually condensed to form the first stars, about 100 million years after the Big Bang. At that time—and for 100 million or so years afterward—there were no heavy elements in the universe. It would require the death of the first generation of stars for the heavier elements, including the carbon and oxygen essential to our beings, to come into existence and begin to accrue in the interstellar gas and debris that could then form solar systems like our own.
The process of nucleosynthesis—to create the heavier elements—is commonly known as nuclear fusion. It is the intensely energetic merging of atomic nuclei to produce a heavier element. In nature, the immense heat and pressure generated by the hyper-intense gravity within stars is what initiates fusion reactions. The only way humans have found to (briefly) sustain fusion reactions is with thermonuclear explosions (hydrogen bombs) where the intense heat from fissioning heavy atoms of uranium or plutonium causes hydrogen to fuse into helium, just as it does amidst the intense heat of stars. (Recently, on a much smaller level, scientists at Lawrence Livermore Laboratory in California successfully used lasers to fuse hydrogen atoms into helium within the confines of a 10 meter wide, concrete-encased target chamber.)
A byproduct of fusion reactions is the release of even more immense energy and it is the force of that energy (like air pumped into a basketball) that supports a star against the weight of its mass. But in the last throes of a star’s life, the eventual depletion of its hydrogen “fuel” (by conversion to helium) causes the star to begin to collapse. The force of gravity simply overpowers the countervailing force of the waning fusion reaction.
Stellar nursery in the Gemini constellations photographed by the Hubble Space Telescope, courtesy NASA/ESA.
In medium-sized stars like our sun, the initiation of the gravitational collapse causes the core of the star to heat up, causing the star to expand into what is known as a red giant. In this process—the beginning of the star’s death throes—the red giant starts fusing helium to carbon, and then carbon to oxygen. Ultimately, the red giant collapses into into a super-dense “white dwarf” while expelling what remains of its outer shell into space as a massive cloud typically rich in carbon, oxygen and nitrogen. For stars like our sun that are relatively isolated in their stellar neighborhoods, the “white dwarf” stage will be the end point. (Not to worry, it’s about 5-6 billion years from now.)
Not all white dwarfs end as white dwarfs though. In the vastness of a universe with a trillion or more galaxies (nearly all of which consist of billions of stars) it is not uncommon for stars to be close enough together to form “binary” systems. If a white dwarf is part of a binary system it can pull in matter from a nearby, companion star. When this happens, the additional matter can make the white dwarf unstable, initiating a runaway fusion reaction of carbon and oxygen in its dense core. This instability triggers what astronomers call a Type 1a supernova—a massive, final explosion that blasts the remnants of the star’s core into space while also creating additional heavy elements including nickel, manganese and iron. Exploding white dwarfs are thought to be a major source—if not the major source—of iron in interstellar space, offering their iron-rich gas to new star and planet formation.
The other main type of exploding stars are Type II supernovae which are triggered by the final explosion of Super red giants—stars that are at least 8 times as massive as our sun but not nearly as common. Also known as “core collapse” supernovae, they mainly forge elements lighter than iron in their last stages, but heavier elements are created by fusion in the inevitable supernova explosion.
One of the remarkable recent discoveries involving the creation of heavy elements occurred just a few years ago thanks, in part, to scientists affiliated with an unusual observatory at the Hanford site in south-central Washington and a twin facility in rural Mississippi. Known as the Laser Interferometer Gravitational-wave Observatories (LIGO), these relatively new instruments (they began operating in 2002) work in tandem to detect gravitational waves rippling across the universe.
Gravitational waves emanate from the most catastrophic celestial events—principally the violent merging black holes and so-called neutron stars. Neutron stars are the collapsed, remnant cores of supergiant stars that remain after supernova explosions. They are so dense that protons and electrons combine under pressure to create a bizarre celestial body that (so far as we can tell) consists largely of subatomic neutrons, hence the term neutron star. The LIGOs and a similar facility in Italy detected gravitational waves from a pair of merging neutron stars in 2017. The convergence produced a brief, colossal flash of light that, when captured and analyzed, revealed the neutron star collision created staggering amounts of heavy elements, including an amount of gold that was several times the mass of the Earth. This was a major discovery, providing evidence that colliding neutron stars are the primary source for most of the heavier elements in the universe.
Events like supernovae and and colliding neutron stars are rare, at least in human terms. The last supernova observed from Earth was nearly 400 years ago. But the resulting outbursts of heavy elements are seminal, at least for us. Just like the primordial first generations of stars, stars forming today don’t need the heavy elements in order to exist. But for us—and other life on Earth—the heavier elements created in the violent death throes of stars are integral to our being.
Apart from the sun (which is only halfway through its lifespan) the nearest star to us is Proxima Centauri. It is 25 trillion miles away. So while we humans may never be able to reach the stars, we are mostly of the stars, a manifestation of a story eminently larger than we are, and one from which we are quite literally made. It’s very hard to get a head around that. But not impossible.
Because our physiology has evolved to rely upon them, humans are incredibly proficient in the way our bodies hoard the heavier elements that come from exploding stars. Ninety percent of our mass comes from elements that didn’t exist before the first stars began to expire.
There are two other key ingredients to this epic: time and distance. Because supernovae happen so infrequently, and so far away, nobody alive today has witnessed a supernova with naked eyes. We are such short-lived beings and yet we consist of essential elements created in dying stars over billions of years. Apart from the sun (which is only halfway through its lifespan) the nearest star to us is Proxima Centauri. It is 25 trillion miles away. So while we humans may never be able to reach the stars, we are mostly of the stars, a manifestation of a story eminently larger than we are, and one from which we are quite literally made. It’s very hard to get a head around that. Though not impossible.
Before researching this piece, I had forgotten the other half of what Carl Sagan said to his Cosmos audience 43 years ago, after he said we are made of “star stuff.” What he added is that we are a way “for the Cosmos to know itself. “ As creatures of star dust, we had evolved not just to ask where we’d come from, but to make remarkable progress toward answering that question.
Thirty four years later, the eminent Jesuit and astrophysicist Rev. George Coyne put it even more eloquently, in a TED Talk entitled: We are all made of stardust. After marveling at how “precious” our tiny planet is, and lauding the progress of modern science in his lifetime (he passed in early 2020 at the age of 87) he said this: “Through physics, biology, chemistry, mathematics we’re able to put the universe in our heads. In us the universe is thinking about itself.”
—tjc
Cover photo, The Pillars of Creation, via the James Webb Telescope, courtesy NASA and the Space Telescope Science Institute (STScI).
Purchase Beautiful Wounds, A Search for Solace and Light in Washington’s Channeled Scablands








Wow,Tim! What a beautiful exposition of our astral origins! But perhaps I think the concept of our origin in the stars is older, such that Joni Mitchell sang in her 1969 song “Woodstock”: “… We are stardust, we are golden. We are billion-year-old carbon”!